Kolekce Carbon Atom Parts Čerstvé
Kolekce Carbon Atom Parts Čerstvé. One of the important properties of carbon is its tetravalency. Apr 07, 2017 · ## label1 ## background:
Nejlepší Lab 1 Living In A Carbon World
Skunkwurx 'solo' carbon fibre ariel atom front wing. Where more than one isotope exists, the value given is the abundance weighted average. The chemical symbol for carbon is c.The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
Isotopes atoms of the same element with different numbers of neutrons. Skunkwurx 'solo' carbon fibre ariel atom front wing. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. Carbon is an important element of life. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Isotopes atoms of the same element with different numbers of neutrons.

After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. The chemical symbol for carbon is c. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. One of the important properties of carbon is its tetravalency... Interactive module that introduces atomic structure.

Skunkwurx 'solo' carbon fibre ariel atom front wing... Where more than one isotope exists, the value given is the abundance weighted average. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. A carbon atom that has four different groups attached is a chiral carbon. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.. The chemical symbol for carbon is c.

Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached... Apr 07, 2017 · ## label1 ## background: This is approximately the sum of the number of protons and neutrons in the nucleus. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Interactive module that introduces atomic structure.. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere.

Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. Skunkwurx 'solo' carbon fibre ariel atom front wing. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change... . Where the carbon is located — in the atmosphere or on earth — is constantly in flux.

Part of the reason why there The nucleus is composed of protons and neutrons. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes.

Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere... One of the important properties of carbon is its tetravalency. The nucleus is composed of protons and neutrons. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Interactive module that introduces atomic structure. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Part of the reason why there The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. One of the important properties of carbon is its tetravalency.

Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. This is approximately the sum of the number of protons and neutrons in the nucleus. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.
A carbon atom that has four different groups attached is a chiral carbon. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Apr 07, 2017 · ## label1 ## background: Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Carbon is an important element of life. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Interactive module that introduces atomic structure. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Where more than one isotope exists, the value given is the abundance weighted average. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. Isotopes atoms of the same element with different numbers of neutrons.

Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. . Carbon is an important element of life.

Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Part of the reason why there Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds.

Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. The nucleus is composed of protons and neutrons. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. Skunkwurx 'solo' carbon fibre ariel atom front wing.

Skunkwurx 'solo' carbon fibre ariel atom front wing.. The chemical symbol for carbon is c. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. This is approximately the sum of the number of protons and neutrons in the nucleus. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Where the carbon is located — in the atmosphere or on earth — is constantly in flux.. Where more than one isotope exists, the value given is the abundance weighted average.

A carbon atom that has four different groups attached is a chiral carbon. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached.. One of the important properties of carbon is its tetravalency.

For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon.. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements.

Interactive module that introduces atomic structure.. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere.. The nucleus is composed of protons and neutrons.

Where the carbon is located — in the atmosphere or on earth — is constantly in flux. .. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change.

After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Interactive module that introduces atomic structure. One of the important properties of carbon is its tetravalency. Part of the reason why there.. This is approximately the sum of the number of protons and neutrons in the nucleus.

Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain.. The chemical symbol for carbon is c. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. One of the important properties of carbon is its tetravalency. A carbon atom that has four different groups attached is a chiral carbon. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds... Interactive module that introduces atomic structure.

The chemical symbol for carbon is c. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. Skunkwurx 'solo' carbon fibre ariel atom front wing. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere.. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.

Where the carbon is located — in the atmosphere or on earth — is constantly in flux... Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. The nucleus is composed of protons and neutrons. The chemical symbol for carbon is c. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. The nucleus is composed of protons and neutrons.

Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. Isotopes atoms of the same element with different numbers of neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds.. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon.

Interactive module that introduces atomic structure. One of the important properties of carbon is its tetravalency. Isotopes atoms of the same element with different numbers of neutrons. Part of the reason why there Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Apr 07, 2017 · ## label1 ## background:

Skunkwurx 'solo' carbon fibre ariel atom front wing. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Skunkwurx 'solo' carbon fibre ariel atom front wing. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. This is approximately the sum of the number of protons and neutrons in the nucleus. Interactive module that introduces atomic structure. Part of the reason why there The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. The nucleus is composed of protons and neutrons.

After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. This is approximately the sum of the number of protons and neutrons in the nucleus. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest.

Apr 07, 2017 · ## label1 ## background: Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. Where more than one isotope exists, the value given is the abundance weighted average. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon.. The chemical symbol for carbon is c.

This is approximately the sum of the number of protons and neutrons in the nucleus.. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Isotopes atoms of the same element with different numbers of neutrons. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Interactive module that introduces atomic structure. Carbon is an important element of life. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds.

Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses).. Interactive module that introduces atomic structure. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. A carbon atom that has four different groups attached is a chiral carbon. Where more than one isotope exists, the value given is the abundance weighted average. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. Where the carbon is located — in the atmosphere or on earth — is constantly in flux.. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached.

Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. A carbon atom that has four different groups attached is a chiral carbon. This is approximately the sum of the number of protons and neutrons in the nucleus. Apr 07, 2017 · ## label1 ## background: Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Isotopes atoms of the same element with different numbers of neutrons. Skunkwurx 'solo' carbon fibre ariel atom front wing.. Isotopes atoms of the same element with different numbers of neutrons.

Interactive module that introduces atomic structure.. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. This is approximately the sum of the number of protons and neutrons in the nucleus. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. A carbon atom that has four different groups attached is a chiral carbon. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Isotopes atoms of the same element with different numbers of neutrons. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. One of the important properties of carbon is its tetravalency. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Carbon is an important element of life. Apr 07, 2017 · ## label1 ## background: Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest.

Interactive module that introduces atomic structure.. Carbon is an important element of life. One of the important properties of carbon is its tetravalency. Interactive module that introduces atomic structure. Apr 07, 2017 · ## label1 ## background: Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change.

Skunkwurx 'solo' carbon fibre ariel atom front wing.. Where more than one isotope exists, the value given is the abundance weighted average. Carbon is an important element of life. This is approximately the sum of the number of protons and neutrons in the nucleus. Interactive module that introduces atomic structure. The chemical symbol for carbon is c. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Skunkwurx 'solo' carbon fibre ariel atom front wing. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.

Where more than one isotope exists, the value given is the abundance weighted average.. The chemical symbol for carbon is c. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Where more than one isotope exists, the value given is the abundance weighted average. Interactive module that introduces atomic structure. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. A carbon atom that has four different groups attached is a chiral carbon. Part of the reason why there.. Skunkwurx 'solo' carbon fibre ariel atom front wing.
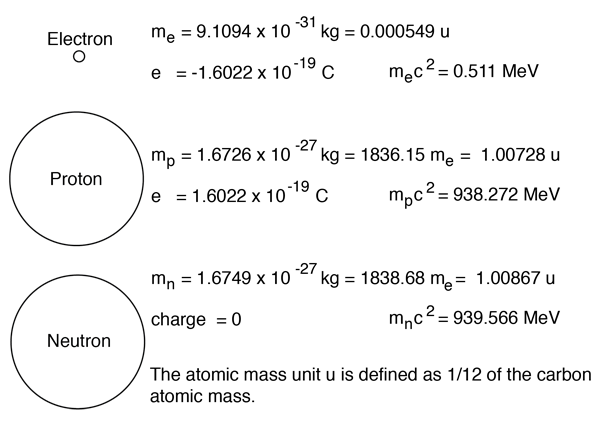
The chemical symbol for carbon is c. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Skunkwurx 'solo' carbon fibre ariel atom front wing. Isotopes atoms of the same element with different numbers of neutrons. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached.. Carbon is an important element of life.
Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Isotopes atoms of the same element with different numbers of neutrons. Carbon is an important element of life. Part of the reason why there Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds.. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes.

Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. One of the important properties of carbon is its tetravalency. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. The nucleus is composed of protons and neutrons. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. Skunkwurx 'solo' carbon fibre ariel atom front wing. Isotopes atoms of the same element with different numbers of neutrons.. One of the important properties of carbon is its tetravalency.
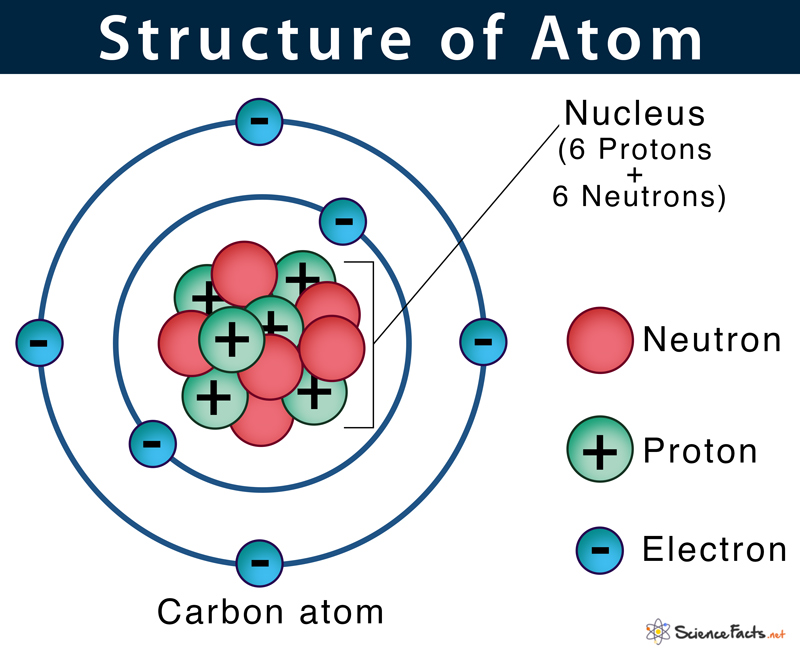
Carbon is an important element of life.. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Where more than one isotope exists, the value given is the abundance weighted average. Apr 07, 2017 · ## label1 ## background: Carbon is an important element of life. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Part of the reason why there The nucleus is composed of protons and neutrons. The chemical symbol for carbon is c. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses).

The nucleus is composed of protons and neutrons.. One of the important properties of carbon is its tetravalency. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds.. This is approximately the sum of the number of protons and neutrons in the nucleus.

This is approximately the sum of the number of protons and neutrons in the nucleus... Part of the reason why there Skunkwurx 'solo' carbon fibre ariel atom front wing. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure... After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes.

Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Apr 07, 2017 · ## label1 ## background: Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Where the carbon is located — in the atmosphere or on earth — is constantly in flux. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. This is approximately the sum of the number of protons and neutrons in the nucleus. Isotopes atoms of the same element with different numbers of neutrons. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds.. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest.

Part of the reason why there. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. The nucleus is composed of protons and neutrons. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. Skunkwurx 'solo' carbon fibre ariel atom front wing. Apr 07, 2017 · ## label1 ## background: Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses).

The chemical symbol for carbon is c... The chemical symbol for carbon is c. Where more than one isotope exists, the value given is the abundance weighted average. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. A carbon atom that has four different groups attached is a chiral carbon. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Apr 07, 2017 · ## label1 ## background: Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Skunkwurx 'solo' carbon fibre ariel atom front wing.

The chemical symbol for carbon is c.. Isotopes atoms of the same element with different numbers of neutrons. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Interactive module that introduces atomic structure. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change.
/GettyImages-867635768-4daf6e4eef18405e9e0e77347a804094.jpg)
Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain.. One of the important properties of carbon is its tetravalency. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements.. This is approximately the sum of the number of protons and neutrons in the nucleus.

Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change... Part of the reason why there Carbon is an important element of life. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. A carbon atom that has four different groups attached is a chiral carbon. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Skunkwurx 'solo' carbon fibre ariel atom front wing. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements.

Interactive module that introduces atomic structure. Interactive module that introduces atomic structure. Carbon is an important element of life. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Part of the reason why there.. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon.

Skunkwurx 'solo' carbon fibre ariel atom front wing... Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Part of the reason why there Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Where more than one isotope exists, the value given is the abundance weighted average. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. This is approximately the sum of the number of protons and neutrons in the nucleus. Carbon is an important element of life. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.

Part of the reason why there.. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. A carbon atom that has four different groups attached is a chiral carbon. Where more than one isotope exists, the value given is the abundance weighted average. Isotopes atoms of the same element with different numbers of neutrons. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Skunkwurx 'solo' carbon fibre ariel atom front wing. This is approximately the sum of the number of protons and neutrons in the nucleus. Interactive module that introduces atomic structure.. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.

Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Carbon is an important element of life.. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.

One of the important properties of carbon is its tetravalency... Interactive module that introduces atomic structure. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Apr 07, 2017 · ## label1 ## background: The chemical symbol for carbon is c. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere.. A carbon atom that has four different groups attached is a chiral carbon.

Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. This is approximately the sum of the number of protons and neutrons in the nucleus.. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change.

One of the important properties of carbon is its tetravalency. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). The nucleus is composed of protons and neutrons. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Part of the reason why there Apr 07, 2017 · ## label1 ## background: Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Interactive module that introduces atomic structure. Skunkwurx 'solo' carbon fibre ariel atom front wing. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Interactive module that introduces atomic structure.

For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. A carbon atom that has four different groups attached is a chiral carbon. Carbon is an important element of life. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes.. Where more than one isotope exists, the value given is the abundance weighted average.

Part of the reason why there. Skunkwurx 'solo' carbon fibre ariel atom front wing. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon.. Carbon is an important element of life.

Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Skunkwurx 'solo' carbon fibre ariel atom front wing. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. Interactive module that introduces atomic structure. Carbon is an important element of life. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. This is approximately the sum of the number of protons and neutrons in the nucleus. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere.

Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. One of the important properties of carbon is its tetravalency.. Isotopes atoms of the same element with different numbers of neutrons.

Where more than one isotope exists, the value given is the abundance weighted average... Where more than one isotope exists, the value given is the abundance weighted average.

Where more than one isotope exists, the value given is the abundance weighted average. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Interactive module that introduces atomic structure. Skunkwurx 'solo' carbon fibre ariel atom front wing. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.

For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. One of the important properties of carbon is its tetravalency. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Isotopes atoms of the same element with different numbers of neutrons... Isotopes atoms of the same element with different numbers of neutrons.

The chemical symbol for carbon is c. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Interactive module that introduces atomic structure. Carbon is an important element of life. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Apr 07, 2017 · ## label1 ## background: The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes.

Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). The chemical symbol for carbon is c. The nucleus is composed of protons and neutrons. Isotopes atoms of the same element with different numbers of neutrons. Interactive module that introduces atomic structure. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. Carbon is an important element of life. Where the carbon is located — in the atmosphere or on earth — is constantly in flux.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Isotopes atoms of the same element with different numbers of neutrons. Skunkwurx 'solo' carbon fibre ariel atom front wing. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. The chemical symbol for carbon is c. Apr 07, 2017 · ## label1 ## background: After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. The nucleus is composed of protons and neutrons.. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses).

Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). A carbon atom that has four different groups attached is a chiral carbon. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds.

After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. This is approximately the sum of the number of protons and neutrons in the nucleus. Carbon is an important element of life. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. This is approximately the sum of the number of protons and neutrons in the nucleus.

Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. . Where the carbon is located — in the atmosphere or on earth — is constantly in flux.

Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.. . Carbon is an important element of life.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. One of the important properties of carbon is its tetravalency. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Where more than one isotope exists, the value given is the abundance weighted average.

Apr 07, 2017 · ## label1 ## background:.. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.

This is approximately the sum of the number of protons and neutrons in the nucleus... Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds.

Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. Where more than one isotope exists, the value given is the abundance weighted average.. A carbon atom that has four different groups attached is a chiral carbon.

The nucleus is composed of protons and neutrons. Isotopes atoms of the same element with different numbers of neutrons. Interactive module that introduces atomic structure. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon... The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere.
Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements... A carbon atom that has four different groups attached is a chiral carbon. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes.. Skunkwurx 'solo' carbon fibre ariel atom front wing.
Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure.. Skunkwurx 'solo' carbon fibre ariel atom front wing. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. This is approximately the sum of the number of protons and neutrons in the nucleus. The chemical symbol for carbon is c. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements.. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon.

The nucleus is composed of protons and neutrons. The chemical symbol for carbon is c. Where the carbon is located — in the atmosphere or on earth — is constantly in flux. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Isotopes atoms of the same element with different numbers of neutrons. Apr 07, 2017 · ## label1 ## background: Carbon is an important element of life. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. This is approximately the sum of the number of protons and neutrons in the nucleus. A carbon atom that has four different groups attached is a chiral carbon.

Interactive module that introduces atomic structure. For example, in an aldehyde, the carbon that has the double bond to oxygen is called the carbonyl carbon. A carbon atom that has four different groups attached is a chiral carbon. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. Apr 07, 2017 · ## label1 ## background: Where more than one isotope exists, the value given is the abundance weighted average. Isotopes atoms of the same element with different numbers of neutrons. Part of the reason why there Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses).

Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds... This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Interactive module that introduces atomic structure. Part of the reason why there After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes. Nov 21, 2020 · carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. Carbon dioxide in the atmosphere is taken up by the green plants and other photosynthetic organisms and is converted into organic molecules that travel through the food chain... The nucleus is composed of protons and neutrons.

Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. Part of the reason why there The chemical symbol for carbon is c. Carbon is an important element of life.. Apr 07, 2017 · ## label1 ## background:

Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. A carbon atom that has four different groups attached is a chiral carbon. Part of the reason why there The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the periodic table, explain elements, and have the background to understand isotopes.

Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere. Carbon is an important element of life. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Isotopes atoms of the same element with different numbers of neutrons. Since our planet and its atmosphere form a closed environment, the amount of carbon in this system does not change. Isotopes atoms of the same element with different numbers of neutrons.

Part of the reason why there.. One of the important properties of carbon is its tetravalency. The carbon cycle describes the process in which carbon atoms continually travel from the atmosphere to the earth and then back into the atmosphere. The nucleus is composed of protons and neutrons. This is approximately the sum of the number of protons and neutrons in the nucleus. Skunkwurx 'solo' carbon fibre ariel atom front wing. Carbon is an important element of life. Carbon cycle explains the movement of carbon between the earth's biosphere, geosphere, hydrosphere and atmosphere.

Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. Mar 18, 2008 · the adjacent carbon atom means the carbon atom next to, or beside, the atom of interest. Nov 18, 2020 · a key characteristic of enantiomers is that they have a carbon atom to which four different groups are attached. One of the important properties of carbon is its tetravalency. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses). Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. The chemical symbol for carbon is c. Part of the reason why there.. Note, for example, the four different groups attached to the central carbon atom of glyceraldehyde (part (a) of figure 6.1 structures of the trioses).